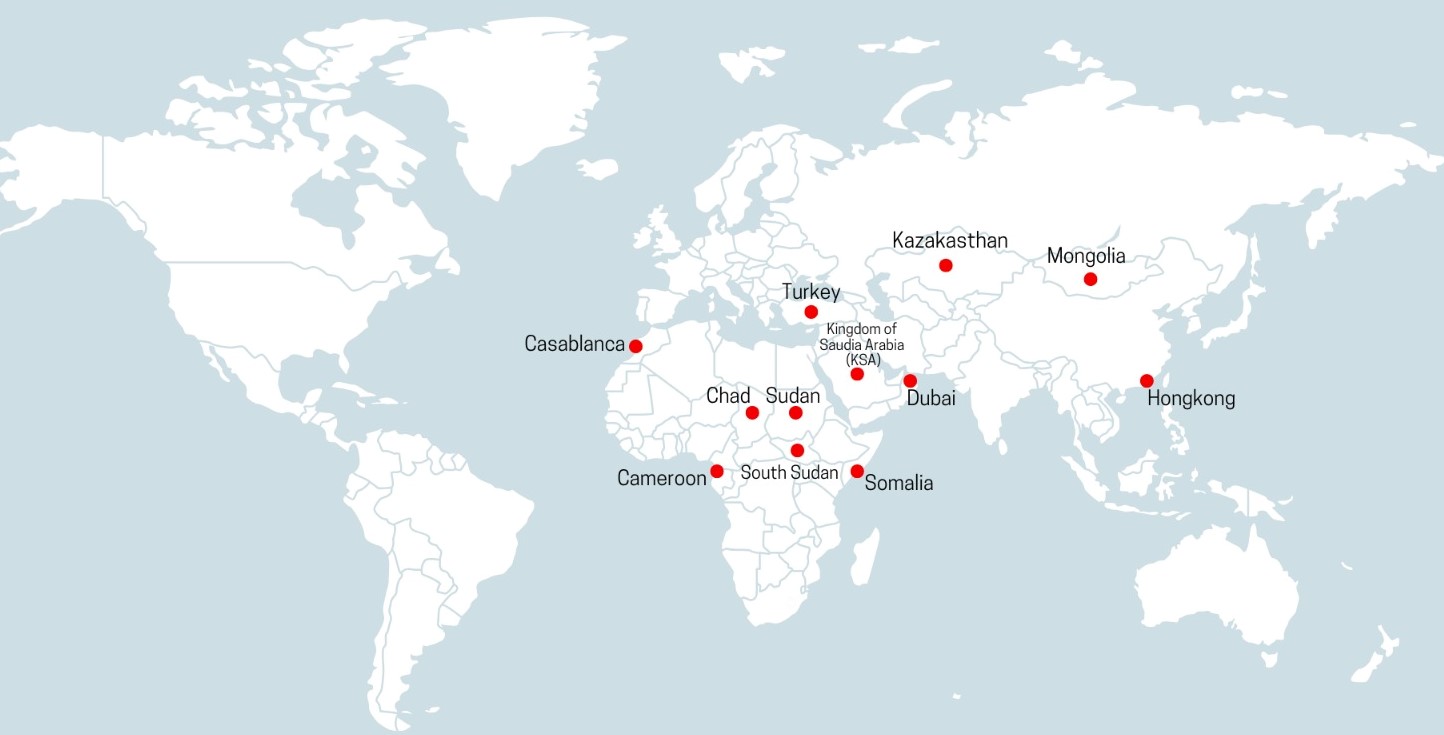
International Location
We at Vecta bio-tech build relationships of trust and growth with partners, distributors and key retailers. Currently with our global footprint across 25 countries, and expertise of over a decade of initiating Pharmaceutical Manufacturing we aim at building engaging connections.
We are sensitive towards the market needs and the regulatory environment of the countries that our partners operate in. There is a key emphasis on being entirely compliant with all the established norms.
GMP Norms
- Local Product registration, where required
- Various International health authority audits
- Various International health authority certificates
All the relevant documents, dossiers and technical support is provided by our dedicated Regulatory Affairs Department to our Importers – Distributors for registrations.
Marketing support is provided for further strengthening the sales in the respective national markets. Considering our expertise and rounded approach our products are reaching across the globe today.

Our Global Presence